PMI’s journey to deliver a smoke-free future
PMI’s journey to deliver a smoke-free future
Philip Morris International (PMI) is widely known as a cigarette company.
But in 2020, we issued our Statement of Purpose, reaffirming our 2016 commitment to deliver a smoke-free future.
We have focused on developing, scientifically substantiating and responsibly commercializing smoke-free products that are less harmful than smoking, with the aim of completely replacing cigarettes as soon as possible.
PMI believes that with the right regulatory encouragement and support from civil society, cigarette sales can end within 10 to 15 years in many countries.
Our innovative alternative products do not burn tobacco or create cigarette smoke, and therefore generate significantly lower levels of toxic substances compared to cigarettes.
We are actively accelerating the decline of cigarette smoking beyond what can be achieved by traditional tobacco control measures alone.
A smoke-free future is within reach, and the benefits this could bring to adults who would otherwise continue to smoke—and therefore to global public health—are enormous.
Philip Morris International (PMI) is widely known as a cigarette company.
But in 2020, we issued our Statement of Purpose, reaffirming our 2016 commitment to deliver a smoke-free future.
We have focused on developing, scientifically substantiating and responsibly commercializing smoke-free products that are less harmful than smoking, with the aim of completely replacing cigarettes as soon as possible.
PMI believes that with the right regulatory encouragement and support from civil society, cigarette sales can end within 10 to 15 years in many countries.
Our innovative alternative products do not burn tobacco or create cigarette smoke, and therefore generate significantly lower levels of toxic substances compared to cigarettes.
We are actively accelerating the decline of cigarette smoking beyond what can be achieved by traditional tobacco control measures alone.
A smoke-free future is within reach, and the benefits this could bring to adults who would otherwise continue to smoke—and therefore to global public health—are enormous.
Why have we staked our future on smoke-free products? Because it’s the right thing to do.
Back in 1997, the UN Focal Point on Tobacco or Health stated that every effort should be made to reduce the toxicity of tobacco products.
We’ve risen to that challenge.
For more than 20 years, we’ve been working on developing and scientifically assessing less-harmful alternatives to cigarettes that do not create smoke, because they do not combust.
Our smoke-free strategy complements efforts by the World Health Organization (WHO) to tackle smoking, and our aspiration is to reduce smoking more than three times faster than the target they have set.

Why have we staked our future on smoke-free products? Because it’s the right thing to do.
Back in 1997, the UN Focal Point on Tobacco or Health stated that every effort should be made to reduce the toxicity of tobacco products.
We’ve risen to that challenge.
For more than 20 years, we’ve been working on developing and scientifically assessing less-harmful alternatives to cigarettes that do not create smoke, because they do not combust.
Our smoke-free strategy complements efforts by the World Health Organization (WHO) to tackle smoking, and our aspiration is to reduce smoking more than three times faster than the target they have set.
“We have shifted our business toward science-based better alternatives.
We look forward to working with decision-makers in governments around the world and organizations such as the WHO to accelerate this transformation.
We remain steadfast in our commitment to unsmoke the world.”
Dr. Moira Gilchrist
Chief Communications Officer,
Philip Morris International

“We have shifted our business toward science-based better alternatives.
We look forward to working with decision-makers in governments around the world and organizations such as the WHO to accelerate this transformation.
We remain steadfast in our commitment to unsmoke the world.”
Dr. Moira Gilchrist
PMI’s Chief Communications Officer
In November 2016, we announced our commitment to a major business transformation—we would build PMI’s future on smoke-free alternatives that are a better choice for adults who would otherwise continue to smoke.
Our scientific results suggested that switching completely to these products would be likely to present less harm than continued smoking.
In December 2016, we submitted an extensive scientific package on our heated tobacco product to the U.S. Food and Drug Administration (FDA) in support of our applications for “modified risk” and “modified exposure” orders.
This marked a huge shift in the history of Philip Morris International, the world’s most successful cigarette company.

In November 2016, we announced our commitment to a major business transformation—we would build PMI’s future on smoke-free alternatives that are a better choice for adults who would otherwise continue to smoke.
Our scientific results suggested that switching completely to these products would be likely to present less harm than continued smoking.
In December 2016, we submitted an extensive scientific package on our heated tobacco product to the U.S. Food and Drug Administration (FDA) in support of our applications for “modified risk” and “modified exposure” orders.
This marked a huge shift in the history of Philip Morris International, the world’s most successful cigarette company.
In January 2018, we presented information related to the modified risk tobacco product applications (MRTPAs) for our heated tobacco product before the Tobacco Products Scientific Advisory Committee at the U.S. Food and Drug Administration (FDA).
During the two-day presentation, we answered the committee’s questions on our research, which suggested that our heated tobacco product was a much better choice for adults who would otherwise continue smoking.
In total, we submitted more than one million pages of documentation related to scientific findings to support our MRTPAs, which, among other things, included aerosol chemistry, non-clinical data, clinical data, consumers studies, and independent studies.
This data package, along with the submissions made in public hearings, was the result of many years of research and development, carried out by hundreds of world-class scientists, engineers, and technicians as part of our rigorous assessment program.
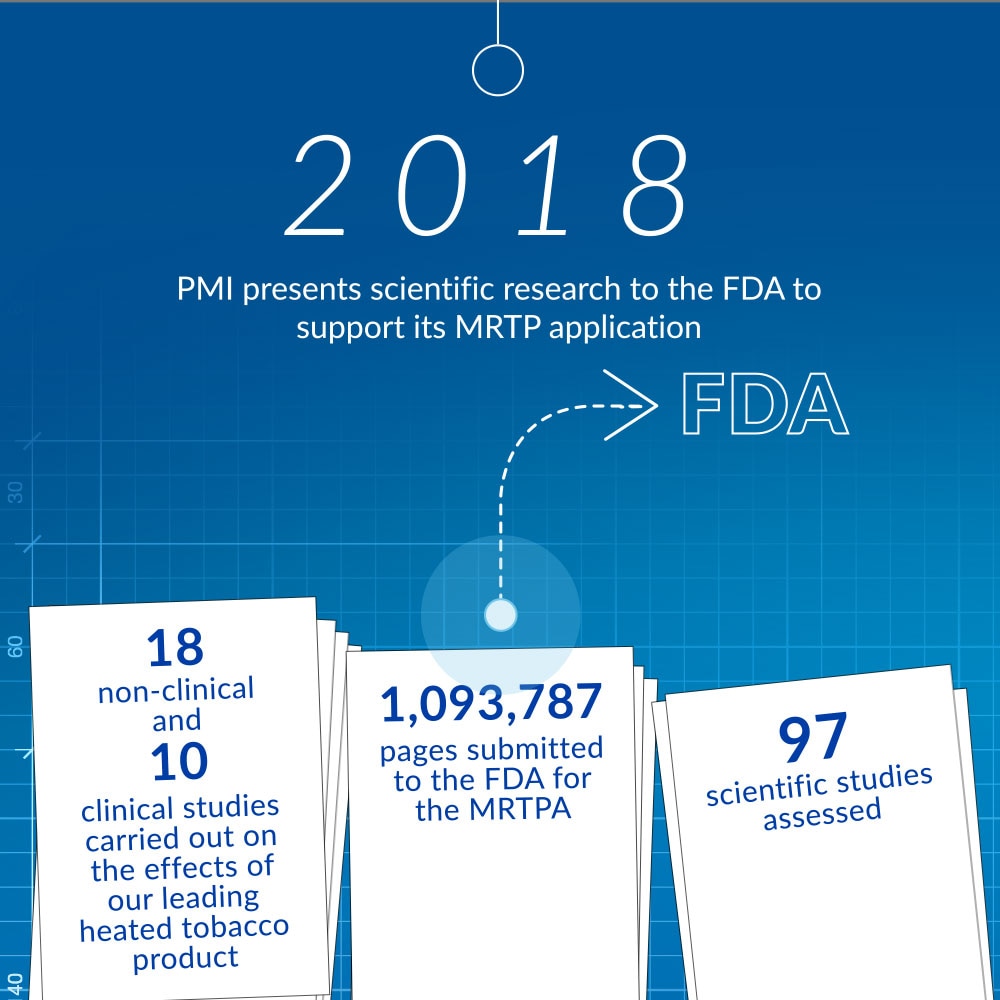
In January 2018, we presented information related to the modified risk tobacco product applications (MRTPAs) for our heated tobacco product before the Tobacco Products Scientific Advisory Committee at the U.S. Food and Drug Administration (FDA).
During the two-day presentation, we answered the committee’s questions on our research, which suggested that our heated tobacco product was a much better choice for adults who would otherwise continue smoking.
In total, we submitted more than one million pages of documentation related to scientific findings to support our MRTPAs, which, among other things, included aerosol chemistry, non-clinical data, clinical data, consumers studies, and independent studies.
This data package, along with the submissions made in public hearings, was the result of many years of research and development, carried out by hundreds of world-class scientists, engineers, and technicians as part of our rigorous assessment program.
In July 2020, the U.S. FDA authorized the marketing of our flagship smoke-free alternative to cigarettes as a modified risk tobacco product (MRTP).
This made it possible to inform American men and women who don’t quit cigarettes that switching completely to our heated tobacco product is a much better choice. The FDA also stated that issuing an exposure modification order for our product was appropriate to promote the public health, and was expected to benefit the health of the population as a whole.
The FDA’s decision provided an important example of how governments and public health organizations can regulate smoke-free alternatives to differentiate them from cigarettes in order to promote the public health.
This decision was consistent with earlier conclusions of other leading regulatory and scientific bodies, including in the U.K., Germany, and the Netherlands, which have found that the product emits lower levels of harmful toxicants.
We believe that comprehensive, science-based regulation can help to rapidly shift adult smokers who would otherwise continue smoking to better alternatives, while simultaneously guarding against unintended consequences.

In July 2020, the U.S. FDA authorized the marketing of our flagship smoke-free alternative to cigarettes as a modified risk tobacco product (MRTP).
This made it possible to inform American men and women who don’t quit cigarettes that switching completely to our heated tobacco product is a much better choice. The FDA also stated that issuing an exposure modification order for our product was appropriate to promote the public health, and was expected to benefit the health of the population as a whole.
The FDA’s decision provided an important example of how governments and public health organizations can regulate smoke-free alternatives to differentiate them from cigarettes in order to promote the public health.
This decision was consistent with earlier conclusions of other leading regulatory and scientific bodies, including in the U.K., Germany, and the Netherlands, which have found that the product emits lower levels of harmful toxicants.
We believe that comprehensive, science-based regulation can help to rapidly shift adult smokers who would otherwise continue smoking to better alternatives, while simultaneously guarding against unintended consequences.
We are striving to become a company that has a net-positive impact on society.
Our strategy is to responsibly transition from a cigarette company into a world-leading smoke-free business, while simultaneously exploring growth opportunities in the wellness and healthcare spaces. Our North Star is to transform for good, to benefit our company, shareholders, consumers, and society.
Voiceover: Jacek Olczak, CEO, Philip Morris International
This company wants to change the world.
If we can get there this is going to be really phenomenal.
Fast-paced music starts
Words on screen read: That’s why at Philip Morris International, we’ve committed to deliver a smoke-free future.
Voiceover: Stefano Volpetti, President, Smoke-Free Inhaled Products & Chief Consumer Officer, PMI.
This company has had the courage, the vision and leadership to establish a new direction.
Voiceover: Bin Li, Chief Operations Services Officer, PMI
It's very difficult for a company to sign up for a big transformation
like this.
Words on screen read: Our ambition? To move away from cigarettes.
Voiceover: Stacey Kennedy, President Americas Region and CEO of PMI’s U.S. business:
We are building our company's future around better alternatives to smoking .
Voiceover: Michael Voegele, Chief Digital and Information Officer, PMI:
I think it's a unique challenge being part of a team that revolutionizes the industry
Words on screen read: Since 2008, we’ve invested over 14 billion dollars in scientific substantiated smoke-free products.
Moira Gilchrist, Chief Communications Officer, speaks:
So , we've become a company that's centered on bringing better options to people.
Voiceover: Jacek Olczak, CEO, Philip Morris International
In just 10 years, we could be saying to people: “Remember when people smoked?”
Words on screen read: By 2030, we aim to have more than two-thirds
of our net revenue come from smoke-free business.
We are currently at 40%.
Source: PMI Q4 Results 2024
Voiceover: Moira Gilchrist, Chief Communications Officer: The facts are out there, and they’re clearly verified and verifiable.
Voiceover: Stefano Volpetti, President, Smoke-Free Inhaled Products and Chief Consumer Officer:
The most important courageous move was the declaration that there is only one way forward: Smoke-free.
Words on screen read: Currently our smoke-free products are available in 95 markets
Source: PMI Q4 Results 2024
Voiceover: Dr Badrul Chowdhury, Chief Life Sciences Officer, Smoke-Free Products:
You actually have a product where the hundred or so chemicals which are known to be harmful are reduced or eliminated. That's a scientific breakthrough.
Voiceover: Dr Moira Gilchrist, Chief Communications Officer, PMI:
This is transforming the entire industry.
Voiceover: Jacek Olczak, CEO, Philip Morris International:
The evidence is clear. Smoke-free alternatives can accelerate the end of smoking.
The world has to move now to help smokers to move to the better alternative.
Words on screen read: 38.6 million adults globally are using our smoke-free products.
Source: PMI Q4 Results 2024
Voiceover: Jacek Olczak, CEO, Philip Morris International:
It's time to make smoking history.
Philip Morris International logo is seen on screen.

































