| INTEGRATED REPORT 2020 |
Topic description
Product reliability means securing the quality and integrity of our products, their components and ingredients, in line with appropriate design and manufacturing standards.
Specifically, for our smoke-free products, it also means ensuring that they reduce risks relative to smoking cigarettes and that the main scientifically substantiated attributes of smoke-free products are upheld.
Product reliability is a tier 2 topic within our strategic pillar Innovating for better products.

Performance metrics
view data
The right thing to do
Cigarette smoking causes serious diseases and is addictive. The best thing is to never start or to quit. While it is the burning of tobacco that creates the vast majority of harmful chemicals, it is the duty of each manufacturer to ensure that—for adult smokers who would otherwise continue to smoke—their respective formulation and design of innovative smoke-free products do not increase the risk to health inherent in cigarette smoking.
For adult smokers who switch to products that do not combust tobacco, it is of paramount importance that these alternatives are both scientifically substantiated to demonstrate they present less risk to health than continued smoking, and are developed, tested, and manufactured in accordance with the applicable regulations and standards to ensure product integrity and quality. Only in this way can smoke-free products positively contribute to the health of consumers who would otherwise be smokers.
The business case
PMI has made it its mission to replace cigarettes with smoke-free products as soon as possible. While smoke-free products are not risk-free, they offer less harmful alternatives to those adult smokers who will not quit nicotine use. Our ambition of a smoke-free future relies on the development and commercialization of products that meet both stringent scientific requirements and technical design criteria.
Smoke-free products must work as intended, and this is ensured through extensive and stringent assessment and controls so that our consumers and stakeholders can have complete confidence in the quality of our products. Investments to maintain and continuously improve the reliability of our products enable us to protect our reputation and brand equity while complying with regulations.
Achieving our aims
Our quality management system (QMS) covers all our manufactured products: Smoke-free, as well as combustible. Our approach to product reliability starts with our farmers and suppliers. We work with them to secure high-quality raw materials, applying robust procurement processes, auditing, and quality procedures. All ingredients and materials we source must comply with applicable regulations, our internal standards, our Responsible Sourcing Principles, and Good Agricultural Practices.

We operate in an extremely competitive and demanding environment where design and innovation must partner commercial and operational excellence to ensure continued market advantage. We need perpetual innovation and to think creatively, commercially, and disruptively about current and next-generation consumer technology devices.
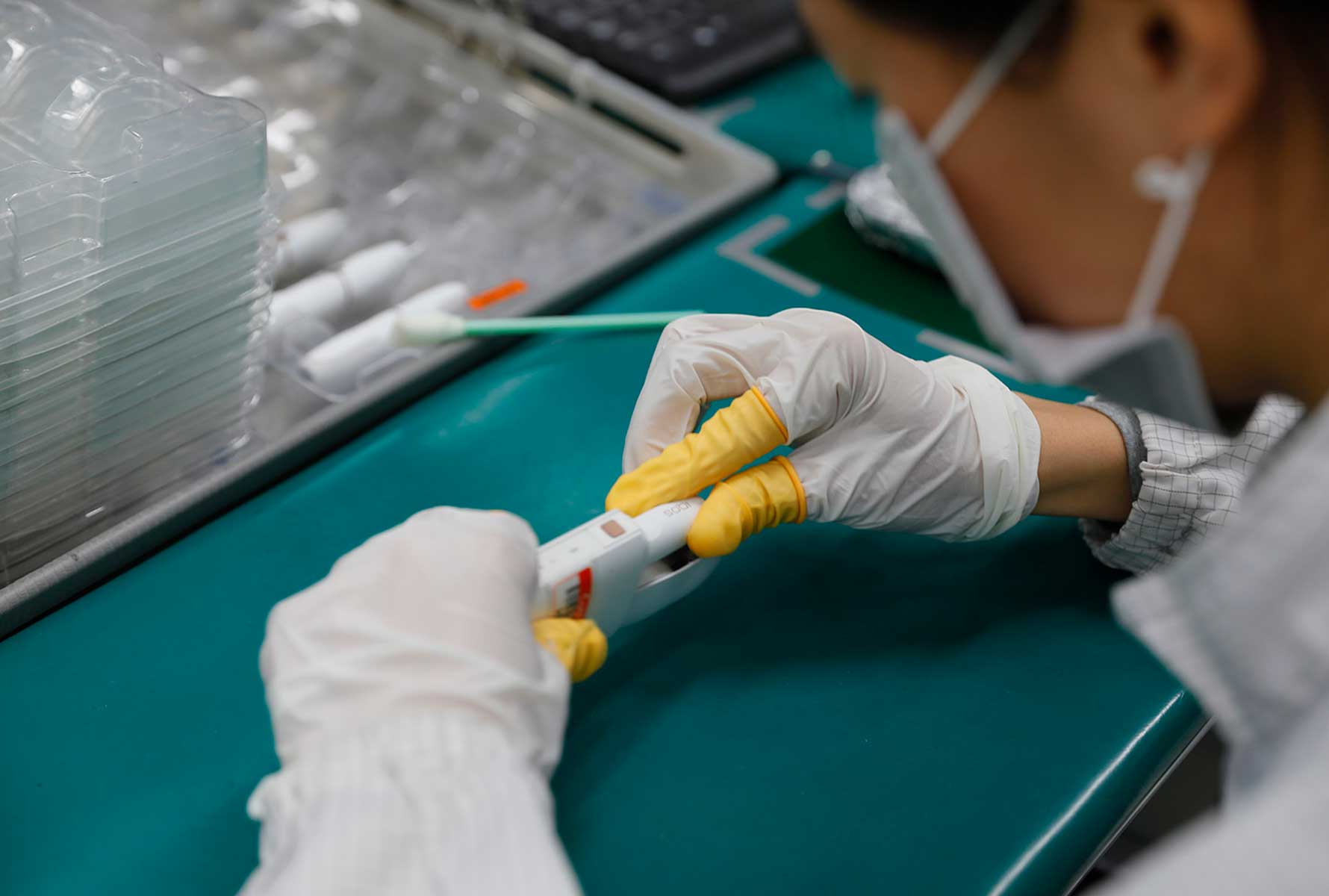
Our factories are all designed to ensure we manufacture our products to the highest quality standards. This includes, for example, the careful selection of materials in contact with the semi-finished and finished products during manufacturing and the delivery of appropriate training to operators. Our manufacturing and quality management systems are subject to inspections by authorities and certified bodies. Globally, a team of around 1,700 product quality assurance professionals ensures that the quality control covers incoming materials, semi-finished components, and finished products across our global footprint. Additionally, we have more than 200 corporate quality professionals, who establish standards, and embed them through our QMS processes. With respect to key electronic suppliers, we have an additional management measure whereby a PMI quality assurance colleague works with each supplier on-site. Toxicological assessment of the heated tobacco products (consumable and electronic devices), as well as cigarettes, is conducted by experienced toxicologists. Compliance with our quality and data standards is monitored using audits by PMI’s Quality Compliance team.
Product reliability extends also to the packaging and transport of finished goods to ensure they reach the consumer in proper condition. Packaging has a key role to play in this regard. It also helps ensure the traceability and identification of our products so we can act promptly should issues arise. Each packaging unit has a unique serial number that allows us to trace the full supply chain from factory to market, enabling backward traceability in the case of product quality issues. Additionally, each device and consumable pack has a unique serial number allowing tracing of the product to the consumer after purchase. In the event of a product recall (of which there were none in 2020), or in case of an identified quality incident, we can leverage this traceability technology to investigate the problem and undertake any necessary actions in an efficient and targeted manner.
Governance and accountability for product integrity and reliability at PMI sits at the highest management level and is guided by a Quality Management System and Internal Standards defined by experienced professionals from different functions. Our work on product integrity and reliability is mainly carried out by teams in our product development, procurement, manufacturing operations, and quality organization departments. Their collective efforts ensure we preserve quality from raw material to the retail shelf.

Our ambition is to deliver highly reliable products based on carefully controlled design verification protocols and rigorous manufacturing standards through process validation. Our commitment to quality aligns with international standards such as ISO. For smoke-free products, it is inspired in part by good manufacturing practices (GMP)—commonly applied in other industries like food or pharmaceuticals.
Smoke-free products
Smoke-free products under our IQOS brand portfolio include heated tobacco products and e-vapor products. Smoke-free systems comprising consumables and electronic devices are subject to strict design control, which ensures that the product is developed according to predefined and controlled criteria demonstrating its reduced toxicity when compared with cigarette smoking. When changes are made to the product, this is done under a strict change management process that assesses the impact of the change on the quality, safety, performance, and regulatory compliance of the product. In addition, as part of product development, we apply use-hazard analysis. We assess the risks related to the intended use and foreseeable misuse of the product and we propose appropriate mitigation measures.
Heated tobacco product devices
The electronic devices for our heat-not-burn product are manufactured according to internal standards and ISO 9001 principles, and certified according to the applicable regulations and standards.
The suppliers of our electronic parts and components of the device, including batteries, must also operate under these quality standards. Particular attention is given to the selection of batteries, with PMI carefully selecting the supplier and performing detailed testing before qualification. Each finished device is subject to rigorous controls before shipment.
In case of consumer complaints regarding the devices, PMI has established a thorough inspection process enabling root cause analysis, which is then used to improve our products and components.
Since the reliability of devices and their lifetime contribute to sustainability outcomes, we strive to strike the right balance between a product’s lifetime in use (and thus lower demand on natural resources) and the process of updating and improving the product’s effectiveness and competitiveness in the market (read more about our efforts in the page on Product eco-design and circularity.
Heated tobacco units
IQOS heated tobacco units are inserted by the consumer into the device holder. The heated tobacco units are composed of a tobacco plug, hollow acetate tube, polymer-film filter, cellulose acetate mouthpiece filter, and filter papers. The uniquely processed tobacco plug is made from a reconstituted blend of high-quality tobacco leaves. We disclose the ingredients of our heated tobacco units on our website.
The manufacturing of heated tobacco units involves high levels of precision and consistency. For instance, the diameter of the unit, as well as the properties of the cast leaf, are crucial to product performance.
Heated tobacco units are also tested for stability, using standard protocols covering different temperature and humidity ranges. The design and packaging aim to preserve the product quality and safety despite various conditions to which the consumer may subject them (read more on PMIScience). This is important as our products are used in different regions of the world; therefore, we need data to demonstrate that the product’s performance will be maintained in various climatic conditions.
E-vapor products
Our e-vapor devices include a cartridge, or tank, containing an e-liquid. The reliability of the product depends on the integrity of the tank; if tampered with, the product could fail. We recognize a trade-off between product reliability and recyclability, but safety of use is our top priority: We are focused on closed tank systems in the development and commercialization of e-vapor products.
We also test our packaging to ensure compliance with the EU Tobacco Products Directive, and we engage with external agencies that test the integrity and safety of e-liquid packaging.
Ingredients in our heated tobacco units and e-liquids
read moreCombustible products
Our combustible products are also subject to strict product development controls. The product specifications and regulations for cigarettes are strictly adhered to, and, at PMI, we apply further internal requirements to ensure the highest quality of our products. We disclose the ingredients used in our conventional products on our website. Such ingredients undergo a toxicological assessment to ensure that their use does not increase the inherent toxicity of cigarette smoke. We keep abreast of strengthening or pending regulation, and a further regulatory compliance assessment is performed to ensure compliance with applicable regulations.
Besides tobacco ingredients, non-tobacco materials such as cigarette paper, filters, and packaging materials are used in our products. Non-tobacco materials also undergo a toxicological assessment and packaging materials are assessed following the main requirements for food contact materials.
We warn consumers about the health effects of all our products. All advertising and consumer packaging for combustible products must contain clear and visible health warnings (read more on our page on Responsible marketing and sales practices).