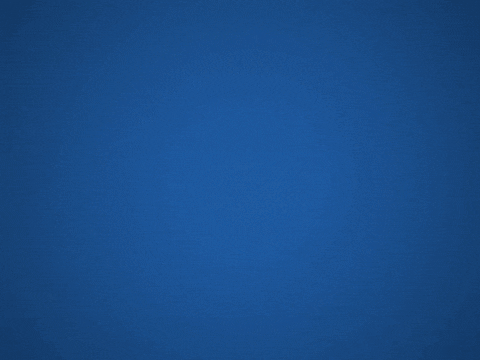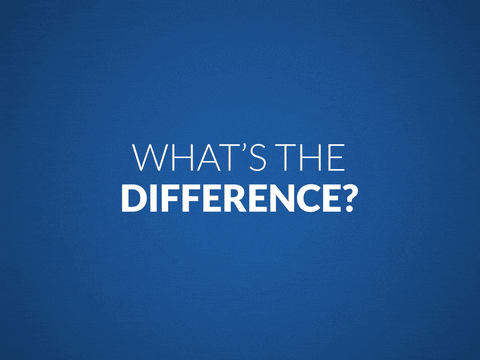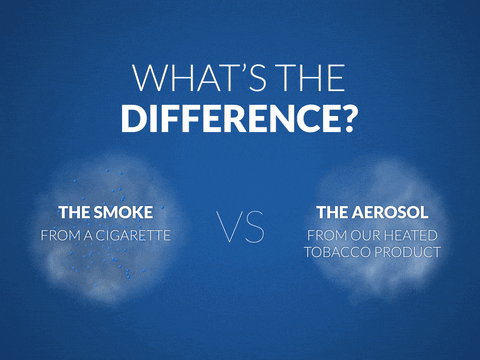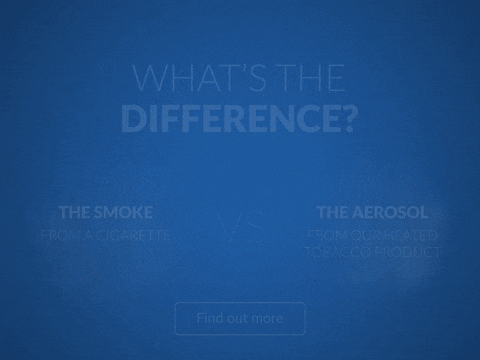
What do PMI scientists do?
This pioneering group of PMI scientists and technicians work collaboratively to create reduced-risk products that mimic the sensory components of cigarettes, without smoke. But what exactly is involved in the scientific process of driving a smoke-free product’s journey from conception to mass production?
It begins with the initial assessment of a product’s risk-reduction potential. This is based on the quality of the product’s design and on strict manufacturing quality controls to ensure it delivers a consistent aerosol. During this early phase, tests are conducted by PMI scientists to determine if the product design will lead to an overall and significant reduction in harmful and potentially harmful constituents (HPHCs) in the aerosol, compared with cigarette smoke.
The science behind PMI’s smoke-free transformation
Background music interspersed with film of PMI scientists working in a laboratory.
Patrick Picavet, Chief Medical Officer, PMI, speaks to camera:
Any manufacturer of a product
has the obligation
to bring data to the table
to show what a product does and what it doesn't do.
Text appears with the title: The science behind PMI’s smoke-free transformation
Emilija Veljkovic,
Manager, Scientific & Medical Affairs, PMI, speaks to camera:
Typically, people are surprised
when they see what type of science we do here in Philip Morris.
Adam Lenart, Manager,
Population and Public Health Research, PMI, speaks to camera:
What we want to understand is how our consumers benefit from our products.
Lindsay Reese, Publications Manager, PMI, speaks to camera:
So we start with analyzing what comes out of the product.
So aerosol chemistry and physics.
And then we test that aerosol on in vitro.
So in cell cultures.
And then
at that point we start moving into clinical data.
Patrick Picavet speaks to camera:
In clinical studies you generate and collect data in a very controlled way.
You bring patients into
the study.
You follow them through the study and you collect the data specifically
for the study.
Animated “Clinical data” infographic appears as Patrick talks, showing how you
create a research question, test on human participants, collect data to create clinical data analysis, which forms clinical evidence.
Christelle Haziza, Global Head, Scientific & Medical Affairs, PMI, speaks to camera:
When it comes to real-world evidence studies,
it’s a little bit different because the population is larger.
Patrick Picavet speaks to camera:
You use data
sources, which are called real-world data sources,
such as electronic records, medical records, health insurance claims, for example.
Animated “Real-world data” infographic appears as Patrick talks,
show how you use electronic medical records, data from digital registries, medical claims data, digital health technologies, questionnaires, and routinely collected data to create real-world data analysis, which forms real-world evidence.
Christelle Haziza speaks to camera:
It's a little bit more representative of what is happening in real-life conditions.
And this is why they are called that way.
Emilija Veljkovic
speaks to camera:
We are taking a very comprehensive approach
in order to understand better our products
and why are they better compared to conventional cigarettes.
Marina
Suvakov, Global Head, Safety Surveillance, PMI, speaks to camera:
That requires a lot of science.
It requires a lot of reports that go to different regulatory bodies.
And all of that really
is to provide information to, most importantly,
our consumers, but also to share with the health agencies.
So when they evaluate the approval of our products, they have all the information
that we
have.
Adam Lenart speaks to camera:
This is the data that our consumers need.
What regulators need, what scientists
and what the public needs.
Patrick Picavet speaks to camera:
The reality is that we have about
1 billion smokers
smoking today
and in the foreseeable future.
For these people
tobacco harm reduction
and smoke-free products
can be a tool to get away
from combustible cigarettes.
The Philip Morris International logo appears.
Measuring toxicity
Another area of research involves measuring the level of toxicity in the aerosol of smoking alternatives compared to cigarette smoke.
PMI carries out these assessments using standard and advanced toxicology methods (comparable to those used in the pharmaceutical industry) to establish whether the reduction in HPHCs leads to a lower impact of the product aerosol on the biological
mechanisms underlying smoking-related diseases.
Next up is the clinical assessment stage, in which PMI scientists study adult smokers to determine if switching to the better alternative to smoking reduces their exposure to harmful compounds.
The effects of using the smoke-free product are assessed against both people who have continued smoking cigarettes and those who have quit tobacco and nicotine completely.
Perception and behavior studies
Then, PMI scientists conduct wider perception and behavior studies to assess a smoke-free product’s potential to benefit public health.
A key area of this is understanding how different groups of people view the risk profile of a smoke-free
product and how likely they are to permanently adopt it instead of smoking cigarettes, while making every effort to ensure that the product does not appeal to non-smokers, former smokers, or those trying to quit nicotine and tobacco products completely.
Long-term assessment
Finally, there’s the long-term assessment stage. This involves monitoring and researching the use of PMI’s smoke-free products once they’re available to buy, in order to assess their contribution to harm reduction. This is achieved by collating qualitative and quantitative data on the use of the smoking alternative in the real world.
At the heart of each phase of product development and testing are the PMI scientists, all of whom are determined to reshape the future of public health.
PMI’s R&D employees are working with painstaking precision and ambition to make a difference to the lives of smokers across the world.
Share this article